
At the time, the process was called imbibition due to the fact that solutes like salt and sugar attracted the water from the material they touched. The ancients observed that adding salt or sugar removes water from tissues. Humans have recognized the potential of osmosis since antiquity, employing it to preserve foods. The same applies for nutrients and minerals, which are also transferred by osmosis. Blood is a more dilute solution than the cell’s cytoplasm, so water will cross through the cell wall. The cell wall acts as a semipermeable membrane, creating osmotic pressure between the inside and outside of the cell. When we drink water, cells absorb it by osmosis just like plant roots. Perhaps a more relatable example is within our own bodies. Roots have hair, which increases surface area and hence the water intake by the plants. Every root acts as a semipermeable barrier, which allows water molecules to transfer from high concentration (soil) to low concentration (roots). Hence, the roots of the plants absorb water and from the roots, water travels to different parts of the plants be it leaves, fruits or flowers. When we water plants, we usually water the stem end and soil in which they are growing. However, if a plant is surrounded by a solution that contains a lower concentration of water, then the water molecules of the solution inside the plant’s cells will be expelled by osmosis, turning the plant flaccid. During this process, the plant cell will become firm. If the plant’s cells are surrounded by a solution that contains a higher concentration of water molecules than the solution inside the cells, water will enter the leaves, fruits, and flowers by osmosis. When we water them, we pour it on the stem end and soil.

Each cell of our body, plants, and animals around us owe their survival to osmosis. Osmosis is one of the essential processes of life. Once the statistical probability of water molecules passing through the membrane is equal, osmosis stops.
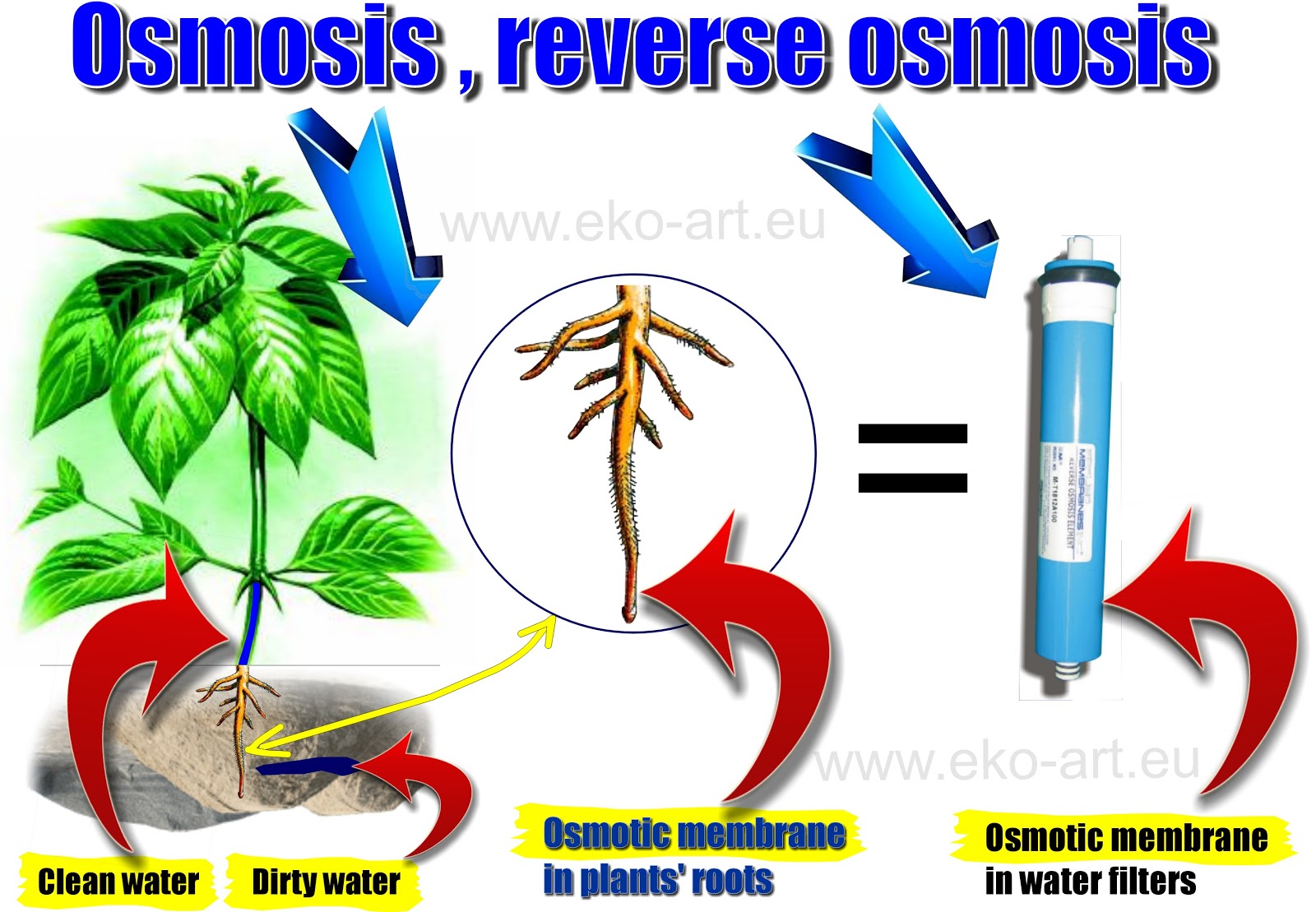
This mismatch means that there’s a greater statistical probability of more water molecules passing through the membrane from a less concentrated solution. Key to osmosis is the presence of a semipermeable membrane that makes it more likely for water molecules in a low concentration solution to collide with the membrane and pass through, whereas water molecules in a concentrated solution will have far fewer molecules of water colliding with the membrane and passing through. It’s the same reason why you should never put a snail near salt, which would cause the poor creature to die as its water is extracted.


 0 kommentar(er)
0 kommentar(er)
